
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k.
ch4(g) + ccl4(g) \rightleftharpoons 2ch2cl2(g)
when 0.31 moles of ccl4(g) are removed from the equilibrium system at constant temperature:
1. the value of
a. increases.
b. decreases.
c. remains the same.
2. the value of qc
a. is greater than
b. is equal to kc.
c. is less than kc.
3. the reaction :
a. run in the forward direction to reestablish equilibrium.
b. run in the reverse direction to reestablish equilibrium.
c. remain the same. it is already at equilibrium.
4. the concentration of ch4 will:
a. increase.
b. decrease.
c. remain the same.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Consider the following system at equilibrium where \delta h° = 18.8 kj, and kc = 9.52×10-2, at 350 k...
Questions

English, 17.12.2020 14:00

World Languages, 17.12.2020 14:00


Physics, 17.12.2020 14:00


Biology, 17.12.2020 14:00



English, 17.12.2020 14:00

Computers and Technology, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00


Mathematics, 17.12.2020 14:00




Mathematics, 17.12.2020 14:00


Social Studies, 17.12.2020 14:00

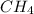 will increase because product will be converted to reactants to reestablish equilibrium.
will increase because product will be converted to reactants to reestablish equilibrium.

