
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not on the reactant concentration. it is expressed as: t1/2=0.693/k
for a second-order reaction, the half-life depends on the rate constant and the concentration of the reactant and so is expressed as: t1/2= 1/k[a]0
a. a certain first-order reaction (a--> products) has a rate constant of 3.30×10^-3 s^-1 at 45 degrees c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?
b. a certain second-order reaction (b--> products) has a rate constant of 1.70×10^-3 m^-1*s^-1 at 27 degrees c and an initial half-life of 296 s. what is the concentration of the reactant b after one half-life?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
For a first-order reaction, the half-life is constant. it depends only on the rate constant and not...
Questions







English, 20.11.2019 01:31




Mathematics, 20.11.2019 01:31


Mathematics, 20.11.2019 01:31



Computers and Technology, 20.11.2019 01:31




![[A_t]=[A_0]e^{-kt}](/tpl/images/0417/6687/1ef89.png)
![[A_t]](/tpl/images/0417/6687/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0417/6687/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0417/6687/0d33c.png) = 0.0625
= 0.0625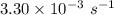
![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0417/6687/16cf4.png)
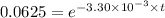
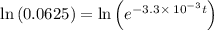
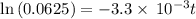
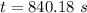
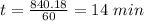
![t_{1/2}=\frac{1}{k[A_o]}](/tpl/images/0417/6687/4d220.png)
![[A_o]](/tpl/images/0417/6687/dc622.png) is the initial concentration = ?
is the initial concentration = ?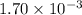 M⁻¹s⁻¹
M⁻¹s⁻¹
![296=\frac{1}{1.70\times 10^{-3}\times [A_o]}](/tpl/images/0417/6687/00cf0.png)
![296=\frac{1000}{1.7[A_o]}](/tpl/images/0417/6687/64d51.png)
![[A_o]=\frac{1250}{629}](/tpl/images/0417/6687/7cd99.png)
![[A_o]=1.99\ M](/tpl/images/0417/6687/59c0f.png)
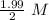 = 0.995 M
= 0.995 M

