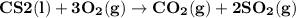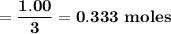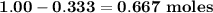Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the following chemical equation. cs2(l) + 3o2(g) → co2(g) + 2so2(g)
a. if 1.00 mol cs2 reacts with 1.00 mol o2, identify the limiting reactant.
b. how many moles of excess reactant remain?
c. how many moles of each product are formed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Select the correct answer from each drop-down menu. daniel and sanya are scientists. daniel is studying whether the increasing frequency of tropical storms is affecting coastal erosion. sanya is investigating whether the discharge from industrial plants has any impact on the ph concentration of freshwater swamps in the surrounding area. which fields of science are daniel’s and sanya’s studies most closely related to? daniel’s field of study is related to science, and sanya’s field of study is related to .
Answers: 3

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Carbon disulfide burns in oxygen to yield carbon dioxide and sulfur dioxide according to the followi...
Questions

Mathematics, 21.05.2021 03:50

Social Studies, 21.05.2021 03:50




Mathematics, 21.05.2021 03:50


Mathematics, 21.05.2021 03:50


Spanish, 21.05.2021 03:50


Mathematics, 21.05.2021 03:50

English, 21.05.2021 03:50



History, 21.05.2021 03:50


Mathematics, 21.05.2021 03:50

Mathematics, 21.05.2021 03:50

Arts, 21.05.2021 03:50