
Chemistry, 14.12.2019 01:31 ariellllllllllllllll
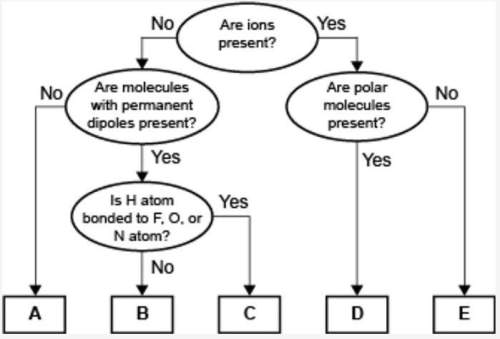
Aconcept map for four types of intermolecular forces and a certain type of bond is shown. an ellipse is shown. inside the ellipse is written are ions present. an arrow from the right side of the ellipse has yes written on it and points to another ellipse. this ellipse has are polar molecules present written inside it. this ellipse has two arrows coming out of it. one arrow has yes written on it and leads to a rectangular box that has d written inside it. the second arrow has no written on it and leads to a rectangular box that has e written inside it. an arrow from the left of the topmost ellipse has no written on it and leads to an ellipse that has are molecules with permanent dipoles present written on it. an arrow that has no written on it, points from this ellipse towards a rectangle that has a written inside it. an arrow that has yes written on it, points from this ellipse towards another ellipse that has is h atom bonded to f, o, or n atom written on it. this ellipse also has two arrows coming from it. the arrow with yes written on it leads to a rectangle that has c written on it and the arrow that has no written on it leads to a rectangle that has b written inside it.
compare the relative strength of the two forces b and c. explain how you determined this comparison by identifying the forces.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Aconcept map for four types of intermolecular forces and a certain type of bond is shown. an ellipse...
Questions

English, 17.05.2021 01:00





English, 17.05.2021 01:00



Chemistry, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00

Chemistry, 17.05.2021 01:00


Mathematics, 17.05.2021 01:00

Biology, 17.05.2021 01:00

Social Studies, 17.05.2021 01:00

History, 17.05.2021 01:00

Mathematics, 17.05.2021 01:00

SAT, 17.05.2021 01:00




