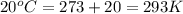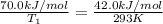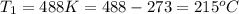Chemistry, 14.12.2019 01:31 ayoismeisjjjjuan
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst. the activation energy of this (uncatalyzed) reaction is 70.0 kj/mol. when the catalyst is added, the activation energy (at 20.ºc) is 42.0 kj/mol. theoretically, to what temperature (ºc) would one have to heat the hydrogen peroxide solution so that the rate of the uncatalyzed reaction is equal to the rate of the catalyzed reaction at 20.ºc? assume the frequency factor a is constant, and assume the initial concentrations are the same. temperature = __ºc

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
Apopular chemical demonstration is the "magic genie" procedure, in which hydrogen peroxide decompose...
Questions

Mathematics, 16.07.2019 16:00


Social Studies, 16.07.2019 16:00



English, 16.07.2019 16:00

History, 16.07.2019 16:00

Health, 16.07.2019 16:00

History, 16.07.2019 16:00


History, 16.07.2019 16:00


History, 16.07.2019 16:00


Social Studies, 16.07.2019 16:00


Mathematics, 16.07.2019 16:00

Mathematics, 16.07.2019 16:00

Biology, 16.07.2019 16:00

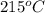
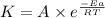
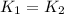
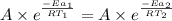
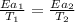 ..........(1)
..........(1)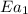 = activation energy for non-catalyzed reaction = 70.0 kJ/mol
= activation energy for non-catalyzed reaction = 70.0 kJ/mol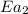 = activation energy for catalyzed reaction = 42.0 kJ/mol
= activation energy for catalyzed reaction = 42.0 kJ/mol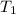 = temperature for non-catalyzed reaction = ?
= temperature for non-catalyzed reaction = ?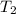 = temperature for catalyzed reaction =
= temperature for catalyzed reaction =