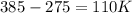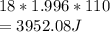Chemistry, 14.12.2019 03:31 louiecampos
Calculate the amount of energy required to increase the temperature of 18.0 go vapor water from 275.0 degrees * c to 385 degrees * c (specific heat of water)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Calculate the amount of energy required to increase the temperature of 18.0 go vapor water from 275....
Questions

Mathematics, 06.05.2020 00:28





Mathematics, 06.05.2020 00:28



Mathematics, 06.05.2020 00:28

Mathematics, 06.05.2020 00:28

History, 06.05.2020 00:28


English, 06.05.2020 00:28

History, 06.05.2020 00:28

Social Studies, 06.05.2020 00:28



Mathematics, 06.05.2020 00:28

English, 06.05.2020 00:28

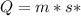 Δ
Δ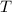
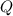 is the heat supplied
is the heat supplied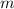 is the mass of the substance (water vapour) = 18g
is the mass of the substance (water vapour) = 18g is the specific heat of the substance ( water vapour) =
is the specific heat of the substance ( water vapour) = 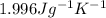 Δ
Δ