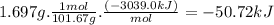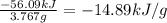Chemistry, 14.12.2019 03:31 mahmudabiazp3ekot
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mol. when 1.697 g of compound a (molar mass = 101.67 g / mol ) is burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.661 ∘ c. what is the heat capacity (calorimeter constant) of the calorimeter? c = kj/°c suppose a 3.767 g sample of a second compound, compound b, is combusted in the same calorimeter, and the temperature rises from 23.23 ∘ c to 27.28 ∘ c. what is the heat of combustion per gram of compound b?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mo...
Questions



Mathematics, 02.10.2020 20:01

Mathematics, 02.10.2020 20:01





Social Studies, 02.10.2020 20:01




English, 02.10.2020 20:01


English, 02.10.2020 20:01





Biology, 02.10.2020 20:01