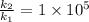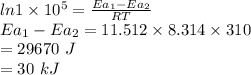Chemistry, 14.12.2019 03:31 Tanija1995
Suppose that a certain biologically important reaction is quite slow at physiological temperature (37 oc) in the absence of a catalyst. assuming arrhenius behavior, by how much must an enzyme lower the activation energy of the reaction to achieve a 1 x 105-fold increase in the reaction rate? (give your answer in kj)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1


Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Suppose that a certain biologically important reaction is quite slow at physiological temperature (3...
Questions

Mathematics, 13.02.2021 19:00


Social Studies, 13.02.2021 19:00

Mathematics, 13.02.2021 19:00

Mathematics, 13.02.2021 19:00

Mathematics, 13.02.2021 19:00

Social Studies, 13.02.2021 19:00



English, 13.02.2021 19:00

History, 13.02.2021 19:00

Business, 13.02.2021 19:00


Mathematics, 13.02.2021 19:00

Business, 13.02.2021 19:00


Mathematics, 13.02.2021 19:00

Chemistry, 13.02.2021 19:00


Mathematics, 13.02.2021 19:10

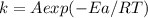
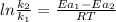
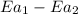 is the lowering in activation energy by enzyme,
is the lowering in activation energy by enzyme,