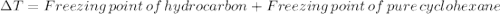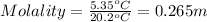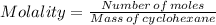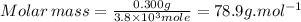Chemistry, 14.12.2019 04:31 Chewbacka2020
Astudent measures the mass of a beaker with an empty test tube in it, and finds that the mass is 156.852 grams. they then add approximately 15 ml of pure cyclohexane to the test tube and reweigh the beaker and test tube with cyclohexane in it. the beaker, test tube and cyclohexane weighs 171.206 grams. then they measure the freezing point of the pure cyclohexane and find that it is 6.60 degree c. the student then adds 0.300 grams of an unknown hydrocarbon to the cyclohexane in the test tube and stirs it until it has dissolved. finally, the freezing point of the cyclohexane and hydrocarbon solution was measured and found to be 1.25 degree c. the freezing point depression constant for cyclohexane is 20.2 degree c/m.
1. what is the change in temperature of the freezing point? delta t of freezing point:
2. what is the molality of the unknown hydrocarbon in the solution? molality of hydrocarbon:
3. what is the mass of cyclohexane in the test tube in kilograms? mass of cyclohexane:
4. how many moles of the unknown hydrocarbon are present in the solution? moles of hydrocarbon:
5. what is the molar mass of the unknown hydrocarbon? molar mass of hydrocarbon:

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
Astudent measures the mass of a beaker with an empty test tube in it, and finds that the mass is 156...
Questions

English, 20.10.2019 10:30

Mathematics, 20.10.2019 10:30


Arts, 20.10.2019 10:30



Physics, 20.10.2019 10:30

Social Studies, 20.10.2019 10:30


Mathematics, 20.10.2019 10:30






Mathematics, 20.10.2019 10:30



Mathematics, 20.10.2019 10:30

Social Studies, 20.10.2019 10:30

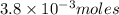
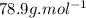 .
.