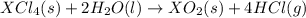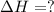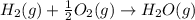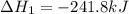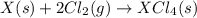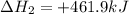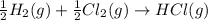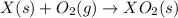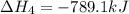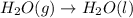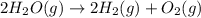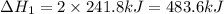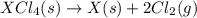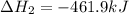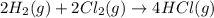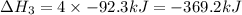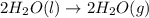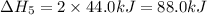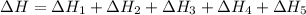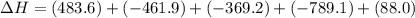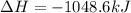Chemistry, 14.12.2019 05:31 ruddymorales1123
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g ) ⟶ h 2 o ( g ) δ h 1 = − 241.8 kj 2 ) x ( s ) + 2 cl 2 ( g ) ⟶ xcl 4 ( s ) δ h 2 = + 461.9 kj 3 ) 1 2 h 2 ( g ) + 1 2 cl 2 ( g ) ⟶ hcl ( g ) δ h 3 = − 92.3 kj 4 ) x ( s ) + o 2 ( g ) ⟶ xo 2 ( s ) δ h 4 = − 789.1 kj 5 ) h 2 o ( g ) ⟶ h 2 o ( l ) δ h 5 = − 44.0 kj what is the enthalpy, δ h , for this reaction? xcl 4 ( s ) + 2 h 2 o ( l ) ⟶ xo 2 ( s ) + 4 hcl ( g )

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Given these reactions, where x represents a generic metal or metalloid 1 ) h 2 ( g ) + 1 2 o 2 ( g )...
Questions


Social Studies, 30.07.2019 21:30

Business, 30.07.2019 21:30



Social Studies, 30.07.2019 21:30

Social Studies, 30.07.2019 21:30










Physics, 30.07.2019 21:30