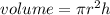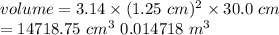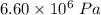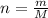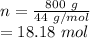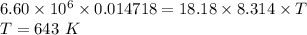Chemistry, 14.12.2019 06:31 Simplytaylorgrenade
Achemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. the vessel is stainless-steel cylinder that measures 25.0 cm wide and 30.0 cm high. the maximum safe pressure inside the vessel has been measured to be 6.60 mpa. for a certain reaction the vessel may contain up to 0.800 kg of carbon dioxide gas.
calculate the maximum safe operating temperature the engineer should recommend for this reaction. write your answer in degrees celsius. round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

You know the right answer?
Achemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reac...
Questions

Mathematics, 19.10.2019 20:50




Mathematics, 19.10.2019 20:50


History, 19.10.2019 20:50




Biology, 19.10.2019 20:50

English, 19.10.2019 20:50


Mathematics, 19.10.2019 20:50


English, 19.10.2019 20:50