Chemistry, 14.12.2019 06:31 2023jpeterson
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
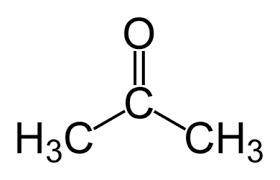
a. in molecule a a central carbon atom is bonded to two c h 3 groups and an o atom through a double bond.
b. the central carbon atom is highlighted.
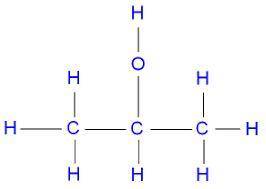
c. in molecule b, a central carbon atom is bonded to two c h 3 groups, an o h group, and an h atom.
d. the central carbon atom is highlighted.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
...
...
Questions



Chemistry, 24.09.2020 08:01

Chemistry, 24.09.2020 08:01


Chemistry, 24.09.2020 08:01

Mathematics, 24.09.2020 08:01

Mathematics, 24.09.2020 08:01

History, 24.09.2020 08:01




Mathematics, 24.09.2020 08:01




Mathematics, 24.09.2020 08:01

Mathematics, 24.09.2020 08:01


Mathematics, 24.09.2020 08:01

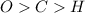
 always gets -1 and
always gets -1 and 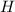 always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.
always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.