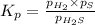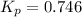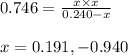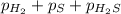Chemistry, 14.12.2019 06:31 nettaboo4664
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g ) − ⇀ ↽ − h 2 ( g ) + s ( g ) h2s(g)↽−−⇀h2(g)+s(g) initially, only h 2 s h2s is present at a pressure of 0.240 0.240 bar in a closed container. what is the total pressure in the container at equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
At a certain temperature, the k p kp for the decomposition of h 2 s h2s is 0.746 0.746 . h 2 s ( g )...
Questions

English, 30.06.2019 01:00








Mathematics, 30.06.2019 01:00


Mathematics, 30.06.2019 01:00


Mathematics, 30.06.2019 01:00

History, 30.06.2019 01:00




Geography, 30.06.2019 01:00


Geography, 30.06.2019 01:00

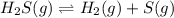
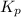 for above equation follows:
for above equation follows: