Chemistry, 14.12.2019 08:31 Nathaliasmiles
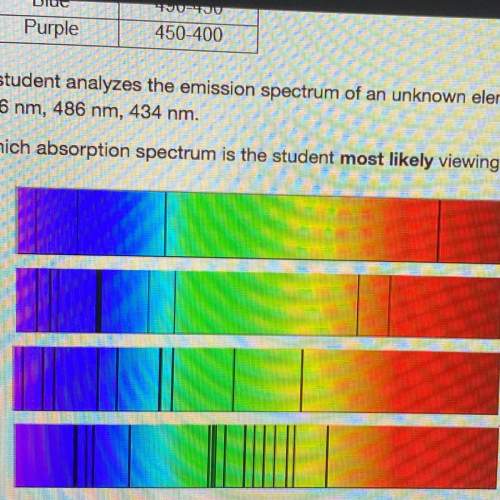
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the following wavelengths:
656 nm, 486 nm, 434 nm.
which absorption spectrum is the student most likely viewing?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Astudent analyzes the emission spectrum of an unknown element and observes strong lines at the follo...
Questions



Mathematics, 22.06.2019 01:30

Mathematics, 22.06.2019 01:30




Computers and Technology, 22.06.2019 01:30

Mathematics, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30


History, 22.06.2019 01:30



Biology, 22.06.2019 01:30