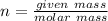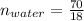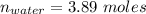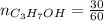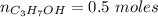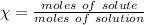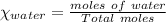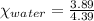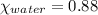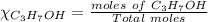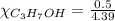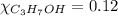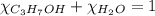Chemistry, 15.12.2019 03:31 jasonoliva13
An aqueous soulution contains 30% c3h7oh and 70% water by mass. what are the mole fractions of each substance in the solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
An aqueous soulution contains 30% c3h7oh and 70% water by mass. what are the mole fractions of each...
Questions





Mathematics, 05.05.2020 21:03

Chemistry, 05.05.2020 21:03

Mathematics, 05.05.2020 21:03

Mathematics, 05.05.2020 21:03


Mathematics, 05.05.2020 21:03

Physics, 05.05.2020 21:03


English, 05.05.2020 21:03




Mathematics, 05.05.2020 21:03



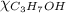 = 0.12
= 0.12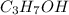 means 30 g of
means 30 g of 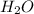 (water) means 70 g of water is present in 100 g of solution
(water) means 70 g of water is present in 100 g of solution