
Chemistry, 16.12.2019 17:31 samjohnson2383
Without doing any calculations, determine the sign of δssys for each of the following chemical reactions.2o3(g)→3o2(g)2h2s(g)+3o2( g)→2h2o(g)+2so2(g)so3(g)+h2o(l)→h2s o4(l)pcl3(l)+cl2(g)→pcl5(s)δssys greater than 0 or δssys smaller than 0

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1
You know the right answer?
Without doing any calculations, determine the sign of δssys for each of the following chemical react...
Questions



Chemistry, 30.12.2020 23:40



English, 30.12.2020 23:40




Social Studies, 30.12.2020 23:40

Mathematics, 30.12.2020 23:40

Health, 30.12.2020 23:40


Computers and Technology, 30.12.2020 23:40






Mathematics, 30.12.2020 23:40

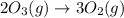 :
: 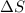 > 0
> 0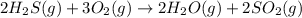 :
: 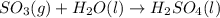 :
: 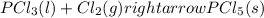 :
: 

