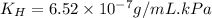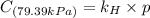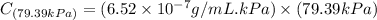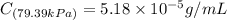Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
Exposing a 250 ml sample of water at 20.∘c to an atmosphere containing a gaseous solute at 27.59 kpa...
Questions

Mathematics, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01



English, 21.10.2020 21:01





Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

Health, 21.10.2020 21:01

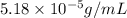
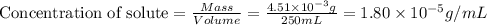
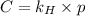
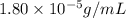
 = Henry's law constant = ?
= Henry's law constant = ?