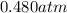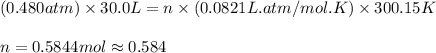Chemistry, 16.12.2019 18:31 Thejollyhellhound20
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.480atm.
calculate the mass and number of moles of carbon dioxide gas that were collected. round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Carbon dioxide gas is collected at 27.0(degrees)c in an evacuated flask with a measured volume of 30...
Questions


Mathematics, 28.08.2020 21:01



Mathematics, 28.08.2020 21:01


Biology, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01

Social Studies, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01

Mathematics, 28.08.2020 21:01


History, 28.08.2020 21:01