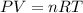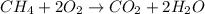Asample of methane gas having a volume of 2.80 l at 25 degree c and 1.65 atm was mixed with a sample of oxygen gas having a volume of 35.0 l at 31 degree c and 1.25 atm. the mixture was then ignited to form carbon dioxide and water. calculate the volume of co_2 formed at a pressure of 2.50 atm and a temperature of 125 degree c.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

You know the right answer?
Asample of methane gas having a volume of 2.80 l at 25 degree c and 1.65 atm was mixed with a sample...
Questions

Chemistry, 05.05.2020 10:09

Social Studies, 05.05.2020 10:09

Mathematics, 05.05.2020 10:09




Social Studies, 05.05.2020 10:09


Mathematics, 05.05.2020 10:09

Mathematics, 05.05.2020 10:09

Mathematics, 05.05.2020 10:09

Mathematics, 05.05.2020 10:09

Mathematics, 05.05.2020 10:09




English, 05.05.2020 10:09



Spanish, 05.05.2020 10:09