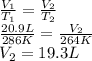An arctic weather balloon is filled with 20.9l of helium gas inside a prep shed. the temperature inside the shed is 13 degree c. the balloon is then taken outside, where the temperature is -9. degree c. calculate the new volume of the balloon. you may assume the pressure on the balloon stays constant at exactly 1atm. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
You know the right answer?
An arctic weather balloon is filled with 20.9l of helium gas inside a prep shed. the temperature ins...
Questions

Arts, 26.01.2021 17:40


Chemistry, 26.01.2021 17:40


Mathematics, 26.01.2021 17:40

History, 26.01.2021 17:40

Advanced Placement (AP), 26.01.2021 17:40






Mathematics, 26.01.2021 17:40



Biology, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40

Mathematics, 26.01.2021 17:40