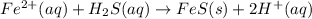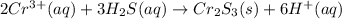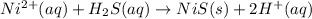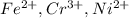Chemistry, 16.12.2019 20:31 genyjoannerubiera
Most sulfide compounds of the transition metals are insoluble in water. many of these metal sulfides have striking and characteristic colors by which we can identify them. therefore, in the analysis of mixtures of metal ions, it is very common to precipitate the metal ions by using dihydrogen sulfate (commonly called hydrogen sulfide), h2s. suppose you had a mixture of fe2 , cr3 , and ni2 . complete the net ionic equations for the precipitation of these metal ions by the use of h2s. (type your answers using the format fe2 for fe2 .)

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
For the following reaction, 5.65 grams of oxygen gas are mixed with excess hydrochloric acid . assume that the percent yield of water is 86.4 %. hydrochloric acid(aq) + oxygen(g) water(l) + chlorine(g) what is the ideal yield of water ? grams what is the actual yield of water ? grams
Answers: 1

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1


Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
Most sulfide compounds of the transition metals are insoluble in water. many of these metal sulfides...
Questions





Mathematics, 17.04.2020 22:20


Mathematics, 17.04.2020 22:20


Mathematics, 17.04.2020 22:20





English, 17.04.2020 22:20