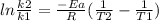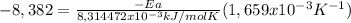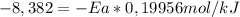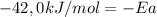Chemistry, 16.12.2019 21:31 krandall232
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction.23.8 kj/mol11.5 kj/mol12.5 kj/mol42.0 kj/mol58.2 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions

Mathematics, 17.07.2019 06:27


Mathematics, 17.07.2019 06:27

Mathematics, 17.07.2019 06:27

Computers and Technology, 17.07.2019 06:27

Computers and Technology, 17.07.2019 06:27

Social Studies, 17.07.2019 06:27

Health, 17.07.2019 06:27

English, 17.07.2019 06:27








History, 17.07.2019 06:27

Chemistry, 17.07.2019 06:27

Chemistry, 17.07.2019 06:27

Biology, 17.07.2019 06:27