
Chemistry, 16.12.2019 21:31 cschellfamily
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set of quantum numbers that does not contain an error. n = 4, l = 4, ml =0 n = 4, l = 0, ml =+1 n = 5, l = 3, ml =-3 n = 3, l = 1, ml = +2 n = 3, l = 2, ml =-3

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Each of the following sets of quantum numbers is supposed to specify an orbital. choose the one set...
Questions









English, 26.02.2020 04:36











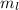 . The value of this quantum number ranges from
. The value of this quantum number ranges from 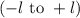 . When l = 2, the value of
. When l = 2, the value of 

