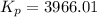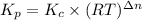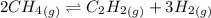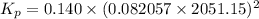Chemistry, 17.12.2019 00:31 jordantay208
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in terms of the concentrations of the gases (in m) or as a function of the partial pressures of the gases (in atmospheres). in the latter case, the equilibrium constant is denoted as kp to distinguish it from the concentration-based equilibrium constant kc (sometimes referenced as just k).for the reaction 2ch4(g)⇌c2h2(g)+3h2(g) kc = 0.140 at 1778 ∘c . what is kp for the reaction at this temperature? express your answer numerically.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
For chemical reactions involving ideal gases, the equilibrium constant k can be expressed either in...
Questions

Mathematics, 12.12.2019 00:31

History, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31






Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

English, 12.12.2019 00:31

Chemistry, 12.12.2019 00:31

Chemistry, 12.12.2019 00:31



Mathematics, 12.12.2019 00:31

Chemistry, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31