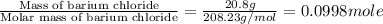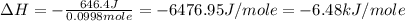Chemistry, 17.12.2019 00:31 raulhill98
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter by the reaction: bacl2 (s) ba2+(aq) + 2cl−(aq) the water is originally at 25.0 °c and after the reaction the temperature of the solution is 26.6 °c. (cs = 4.04 j/(g°c) for the solution). what is the enthalpy change (δh) associated with the reaction as written?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1

Chemistry, 23.06.2019 13:20
Which kind of weather usually forms over the northwest united states in the summer because of maritime polar air masses? 1 )fog 2)dry heat 3) heavy snow 4) heavy rain
Answers: 3
You know the right answer?
An amount of solid barium chloride, 20.8 g, is dissolved in 100 g water in a coffee-cup calorimeter...
Questions

Advanced Placement (AP), 28.08.2019 14:30


Health, 28.08.2019 14:30



Biology, 28.08.2019 14:30

English, 28.08.2019 14:30


History, 28.08.2019 14:30

Physics, 28.08.2019 14:30

Chemistry, 28.08.2019 14:30

Mathematics, 28.08.2019 14:30

Mathematics, 28.08.2019 14:30





Mathematics, 28.08.2019 14:30


Mathematics, 28.08.2019 14:30


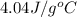
 = final temperature =
= final temperature = 
 = initial temperature =
= initial temperature = 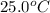

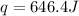
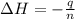
 = enthalpy change = ?
= enthalpy change = ?