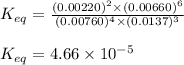9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. without a catalyst, a different reaction predominates: 4nh3(g) + 3o2(g) ⇔ 2n2(g) + 6h2o(g) when 0.0120 mol gaseous nh3 and 0.0170 mol gaseous o2 are placed in a 1.00 l container at a certain temperature, the n2 concentration at equilibrium is 2.20×10-3 m. calculate keq for the reaction at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1
You know the right answer?
9. the first step in industrial nitric acid production is the catalyzed oxidation of ammonia. withou...
Questions


Mathematics, 18.12.2020 18:00



English, 18.12.2020 18:00

Mathematics, 18.12.2020 18:00



Biology, 18.12.2020 18:00

Mathematics, 18.12.2020 18:00



English, 18.12.2020 18:00




Mathematics, 18.12.2020 18:00

Mathematics, 18.12.2020 18:00


Advanced Placement (AP), 18.12.2020 18:00

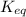 is
is 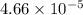
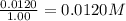
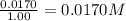
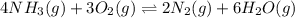
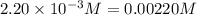
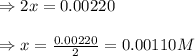
![0.0120-4x=[0.0120-(4\times 0.00110)]=0.00760M](/tpl/images/0421/4577/5e570.png)
![0.0170-3x=[0.0170-(3\times 0.00110)]=0.0137M](/tpl/images/0421/4577/b5ac6.png)
![6x=[6\times 0.00110]=0.00660M](/tpl/images/0421/4577/d0282.png)
![K_{eq}=\frac{[N_2]^2\times [H_2O]^6}{[NH_3]^4\times [O_2]^3}](/tpl/images/0421/4577/7f887.png)