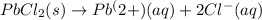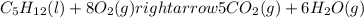Chemistry, 17.12.2019 03:31 20warriorsoul14
For which of the following processes will \deltaδs be negative? pbcl2(s) = pb2+(aq) + 2 cl-(aq)mgo(s) + co2(g) = mgco3(s)co2(aq) = co2(g)c5h12(l) + 8 o2(g) = 5 co2(g) + 6 h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
For which of the following processes will \deltaδs be negative? pbcl2(s) = pb2+(aq) + 2 cl-(aq)mgo(s...
Questions


Social Studies, 17.02.2021 01:50


Spanish, 17.02.2021 01:50

Mathematics, 17.02.2021 01:50



Social Studies, 17.02.2021 01:50

Computers and Technology, 17.02.2021 01:50




History, 17.02.2021 01:50

Mathematics, 17.02.2021 01:50

Chemistry, 17.02.2021 01:50

Mathematics, 17.02.2021 01:50




Computers and Technology, 17.02.2021 01:50

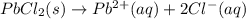 :
: 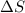 = +ve
= +ve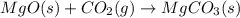 :
: 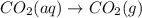 :
: 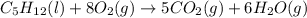 :
: