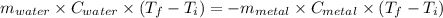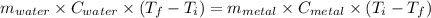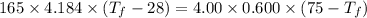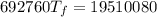Chemistry, 17.12.2019 04:31 aaronjcerrato
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then dropped into 165 g of water in a calorimeter. what is the final temperature of the water if the initial temperature is 28 degrees celcius? the specific heat capacity of water is 4.184 j/g.°c.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
A4.00 g sample of a metal (specific heat = 0.600 j g-1°c-1 is heated to 75 degrees celcius and then...
Questions


Computers and Technology, 23.11.2019 03:31





Physics, 23.11.2019 03:31

Physics, 23.11.2019 03:31

Physics, 23.11.2019 03:31


Physics, 23.11.2019 03:31

Biology, 23.11.2019 03:31


Physics, 23.11.2019 03:31

Physics, 23.11.2019 03:31

Geography, 23.11.2019 03:31

Business, 23.11.2019 03:31

History, 23.11.2019 03:31

Business, 23.11.2019 03:31