Chemistry, 17.12.2019 04:31 coolquezzie
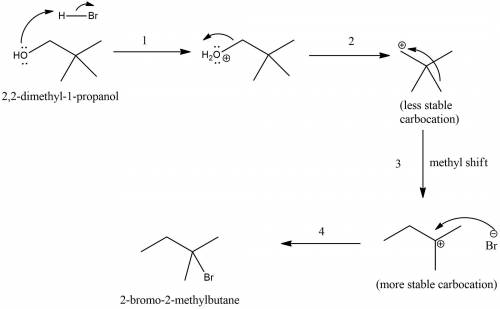
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as the major product. give a mechanistic explanation for these observations. select all that apply. stereoelectronic effects result in an antic op lanar rearrangement of the carbon skeleton. steric hindrance prevents nucleophilic attack. the mechanism requites the development of an unstable positively charged species in the transition state. the mechanism results in a carbocation rearrangement in which a methyl shift occurs. the mechanism requires dissociation of a poor leaving group.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
The reaction of 2,2-dimethyl-1-piopanol with hbr is very slow and gives 2-bromo- 2-methyibutane as t...
Questions







Mathematics, 10.03.2020 07:40


Biology, 10.03.2020 07:40

Mathematics, 10.03.2020 07:40

Advanced Placement (AP), 10.03.2020 07:40

Mathematics, 10.03.2020 07:40

Mathematics, 10.03.2020 07:40


Chemistry, 10.03.2020 07:40

Mathematics, 10.03.2020 07:40

Business, 10.03.2020 07:40


Computers and Technology, 10.03.2020 07:40

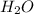 .
.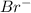 attacks the stable carbocation to produce 2-bromo-2-methylbutane.
attacks the stable carbocation to produce 2-bromo-2-methylbutane.