Chemistry, 17.12.2019 04:31 princessx7543
Given that  [br(g)] = 111.9 kj ⋅ mol⁻¹
[br(g)] = 111.9 kj ⋅ mol⁻¹ [br(g)] = 111.9 kj⋅mol⁻¹
[br(g)] = 111.9 kj⋅mol⁻¹ [c(g)] = 716.7 kj ⋅ mol⁻¹
[c(g)] = 716.7 kj ⋅ mol⁻¹ [c(g)] = 716.7 kj⋅mol⁻¹
[c(g)] = 716.7 kj⋅mol⁻¹ [cbr₄(g)] = 29.4 kj ⋅ mol⁻¹
[cbr₄(g)] = 29.4 kj ⋅ mol⁻¹ [cbr₄(g)] = 29.4 kj⋅mol⁻¹ calculate the average molar bond enthalpy of the carbon–bromine bond in a cbr₄ molecule.
[cbr₄(g)] = 29.4 kj⋅mol⁻¹ calculate the average molar bond enthalpy of the carbon–bromine bond in a cbr₄ molecule.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Given that [tex]\delta h^o_f[/tex] [br(g)] = 111.9 kj ⋅ mol⁻¹[tex]\delta h^o_f[/tex] [br(g)] = 111.9...
Questions

Biology, 10.03.2020 01:03



English, 10.03.2020 01:03










History, 10.03.2020 01:03


Social Studies, 10.03.2020 01:03




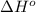
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0421/8523/45485.png)
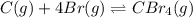
![\Delta H^o_{rxn}=[(n_{(CBr_4)}\times \Delta H^o_f_{(CBr_4)})]-[(n_{(Br)}\times \Delta H^o_f_{(Br)})+(n_{(C)}\times \Delta H^o_f_{(C)})]](/tpl/images/0421/8523/304b7.png)
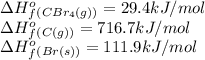
![\Delta H^o_{rxn}=[(1\times 29.4)]-[(4\times 111.9)+(1\times 716.7)]=-1134.9kJ/mol](/tpl/images/0421/8523/33ec8.png)
![\Delta H=-[4\times B.E_{C-Br}]](/tpl/images/0421/8523/b980a.png)
![-1134.9=-[4\times B.E_{C-Br}]](/tpl/images/0421/8523/74d8f.png)