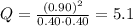Can be monitored visually by following changes in the color of the reaction mixture (br2 is reddish brown, and h2 and hbr are colorless). a gas mixture is prepared at 700 k, in which 0.40 atm is the initial partial pressure of both h2 and br2 and 0.90 atm is the initial partial pressure of hbr. the color of this mixture then fades as the reaction progresses toward equilibrium. give a condition that must be satis- fied by the equilibrium constant k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Can be monitored visually by following changes in the color of the reaction mixture (br2 is reddish...
Questions

History, 20.05.2020 05:57

History, 20.05.2020 05:57


Social Studies, 20.05.2020 05:57

Mathematics, 20.05.2020 05:57



Biology, 20.05.2020 05:57

Spanish, 20.05.2020 05:57


Mathematics, 20.05.2020 05:57


English, 20.05.2020 05:57


Mathematics, 20.05.2020 05:57

Spanish, 20.05.2020 05:57

Mathematics, 20.05.2020 05:57


History, 20.05.2020 05:57

Biology, 20.05.2020 05:57

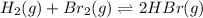
![K_{eq} = \frac{[HBr]^2}{[H_2][Br_2]}](/tpl/images/0422/0071/178f0.png)
![[H_2]_{eq} = [Br_2]_{eq} = 0.40 atm - x, [HBr]_{eq} = 0.90 atm + 2x](/tpl/images/0422/0071/e6f1b.png)