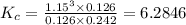Chemistry, 17.12.2019 05:31 Shadow0202
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperatures. ch₄ + h₂ ⇌ 3h₂ + what is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: ch₄, 0.126 m; h₂o, 0.242 m; co, 0.126 m; h₂ 1.15 m, at a temperature of 760 °c?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperature...
Questions


Mathematics, 21.10.2020 22:01

English, 21.10.2020 22:01





Mathematics, 21.10.2020 22:01


Business, 21.10.2020 22:01


Spanish, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

History, 21.10.2020 22:01


Mathematics, 21.10.2020 22:01


Mathematics, 21.10.2020 22:01

![[CH_4]=0.126\ M](/tpl/images/0421/9784/de111.png)
![[H_2O]= 0.242\ M](/tpl/images/0421/9784/d80cb.png)
![[CO]= 0.126\ M](/tpl/images/0421/9784/495e0.png)
![[H_2]= 1.15\ M](/tpl/images/0421/9784/0632f.png)
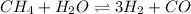
![K_{c}=\frac {\left [ H_2 \right ]^3\left [ CO \right ]}{\left [ CH_4 \right ]\left [ H_2O \right ]}](/tpl/images/0421/9784/6aeab.png)