
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment, 0.30mol of co2 was produced from the reaction of 0.05mol of c6h12o6 with excess o2. the reaction was repeated at the same temperature and in the same container, but this time 0.60mol of co2 was produced. which of the following must be true

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
The reaction between c6h12o6 and o2 is represented by the balanced equation above. in an experiment,...
Questions








Advanced Placement (AP), 27.11.2019 23:31

Computers and Technology, 27.11.2019 23:31








Chemistry, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31


Mathematics, 27.11.2019 23:31

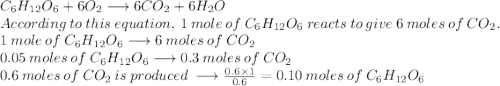
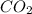 is produced when 0.1 mol of
is produced when 0.1 mol of 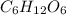 reacts with excess oxygen.
reacts with excess oxygen.

