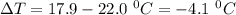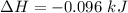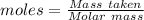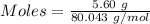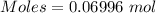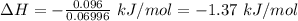Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 is dissolved in 100g of water at 22.0c; the temperature falls to 17.9c. assuming that the specific heat capacity of the solution is 4.18 j/(g*k), calculate the enthalpy of dissolution of nh4no3, in kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Commercial cold packs consist of solid nh4no3 and water. in a coffee-cup calorimeter, 5.60g nh4no3 i...
Questions

History, 01.02.2020 18:45






Mathematics, 01.02.2020 18:45

Biology, 01.02.2020 18:45


Mathematics, 01.02.2020 18:46

History, 01.02.2020 18:46


English, 01.02.2020 18:46

English, 01.02.2020 18:46



History, 01.02.2020 18:46

Mathematics, 01.02.2020 18:46


Mathematics, 01.02.2020 19:42

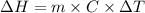
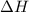 is the enthalpy of dissolution of [tex[NH_4NO_3[/tex]
is the enthalpy of dissolution of [tex[NH_4NO_3[/tex] is the temperature change
is the temperature change