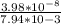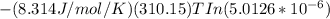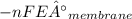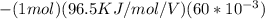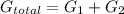Chemistry, 17.12.2019 06:31 rostecorralmart
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreting cells. the cells secrete hcl, proteolytic enzyme zymogens, mucin, and intrinsic factor. the ph of gastric juice is acidic, between 1-3. if the ph of gastric juice is 2.1, what is the amount of energy (? g) required for the transport of hydrogen ions from a cell (internal ph of 7.4) into the stomach lumen? assume that the potential difference across the membrane separating the cell and the interior of the stomach is �60.0 mv (inside of cells negative relative to the lumen of the stomach).
assume that the temperature is 37 �c.
the faraday constant is 96.5 kj�v�1�mol�1 and the gas constant is 8.314� 10�3 kj�mol�1�k�1. express your answer in kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2
You know the right answer?
Gastric juice is made up of substances secreted from parietal cells, chief cells, and mucous-secreti...
Questions

Mathematics, 12.06.2020 22:57



Mathematics, 12.06.2020 22:57


History, 12.06.2020 22:57


Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57


History, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57

Chemistry, 12.06.2020 22:57






English, 12.06.2020 22:57

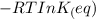
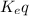 = equilibrum constant for the process
= equilibrum constant for the process
![\frac{[H^+]_(cell)}{[H^+(stomach lumen)]}](/tpl/images/0422/0463/00489.png)
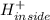 ⇒
⇒ 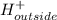
![K_{eq}=\frac{[H^+]_{outside}}{[H^+]_{inside}}](/tpl/images/0422/0463/bf29c.png)
![=\frac{[H^+]_{cell}}{[H^+]_{stomach lumen}}](/tpl/images/0422/0463/cc76d.png)
![[H^+]_{cell}](/tpl/images/0422/0463/36d6e.png) = 10⁻⁷⁴
= 10⁻⁷⁴ ![[H^+]_{stomach lumen}](/tpl/images/0422/0463/f5443.png) = 10⁻²¹
= 10⁻²¹![K_{eq}=\frac{[H^+]_{cell}}{[H^+]_{stomachlumen}}](/tpl/images/0422/0463/c2f36.png)