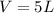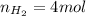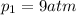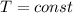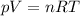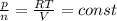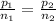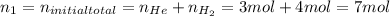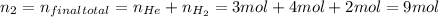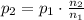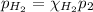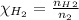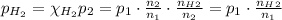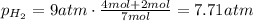Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
A5l container contains 3 moles of helium and 4 moles of hydrogen at a pressure of 9 atms maintaining...
Questions

Mathematics, 18.05.2021 22:00





Mathematics, 18.05.2021 22:00




Mathematics, 18.05.2021 22:00

Mathematics, 18.05.2021 22:00




Mathematics, 18.05.2021 22:00

Social Studies, 18.05.2021 22:00

Spanish, 18.05.2021 22:00


Mathematics, 18.05.2021 22:00

Mathematics, 18.05.2021 22:00