
Chemistry, 17.12.2019 09:31 queenpaige2015
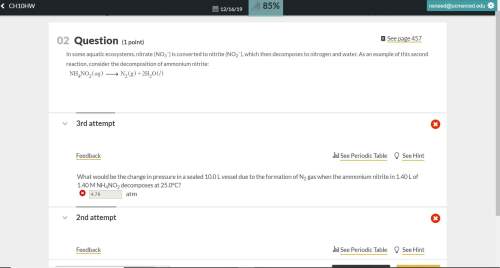
What would be the change in pressure in a sealed 10.0 l vessel due to the formation of n2 gas when the ammonium nitrite in 1.40 l of 1.40 m nh4no2 decomposes at 25.0°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
What would be the change in pressure in a sealed 10.0 l vessel due to the formation of n2 gas when t...
Questions

Physics, 21.06.2019 23:40

Mathematics, 21.06.2019 23:40

Mathematics, 21.06.2019 23:40

Biology, 21.06.2019 23:50

Mathematics, 21.06.2019 23:50


Mathematics, 21.06.2019 23:50



History, 21.06.2019 23:50

History, 21.06.2019 23:50




History, 21.06.2019 23:50

Mathematics, 21.06.2019 23:50


History, 21.06.2019 23:50


Health, 21.06.2019 23:50

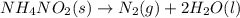
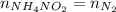
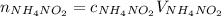
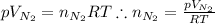
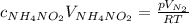
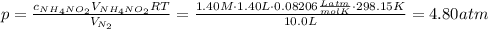
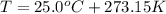 .
.

