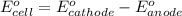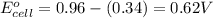Chemistry, 17.12.2019 19:31 Lindseycline123
No−3(aq)+4h+(aq)+3e−→no(g)+2h2o(l)e ∘=0.96v clo2(g)+e−→clo2−(aq)e∘=0.95v cu2+(aq)+2e−→cu(s)e∘=0.34v 2h+(aq)+2e−→h2(g)e∘=0.00v pb2+(aq)+2e−→pb(s)e∘=−0.13v fe2+(aq)+2e−→fe(s)e∘=−0.45v part a use appropriate data to calculate e∘cell for the reaction. 3cu(s)+2no−3(aq)+8h+(aq)→3cu2+(aq)+ 2no(g)+4h2o(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
No−3(aq)+4h+(aq)+3e−→no(g)+2h2o(l)e ∘=0.96v clo2(g)+e−→clo2−(aq)e∘=0.95v cu2+(aq)+2e−→cu(s)e∘=0.34v...
Questions


Mathematics, 05.04.2021 05:00


Mathematics, 05.04.2021 05:00

World Languages, 05.04.2021 05:00


Geography, 05.04.2021 05:00

Mathematics, 05.04.2021 05:00



Geography, 05.04.2021 05:00

Mathematics, 05.04.2021 05:00

Mathematics, 05.04.2021 05:00


English, 05.04.2021 05:00

Geography, 05.04.2021 05:00


Physics, 05.04.2021 05:00


Mathematics, 05.04.2021 05:00

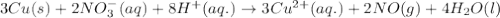
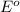 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.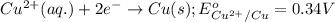 ( × 3 )
( × 3 )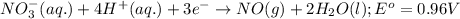 ( × 2 )
( × 2 )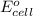 of the reaction, we use the equation:
of the reaction, we use the equation: