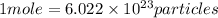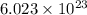Chemistry, 21.08.2019 23:40 thestuckone
Magnesium and iron are metallic elements. how does a mole of magnesium compare with a mole of iron?
a) a mole of iron has more atoms.
b) they both have the same mass.
c) a mole of magnesium has more mass.
d) they both have the same number of atoms.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Magnesium and iron are metallic elements. how does a mole of magnesium compare with a mole of iron?...
Questions

Social Studies, 18.08.2019 09:30

English, 18.08.2019 09:30

Mathematics, 18.08.2019 09:30


Mathematics, 18.08.2019 09:30


History, 18.08.2019 09:30

Computers and Technology, 18.08.2019 09:30

Mathematics, 18.08.2019 09:30

Biology, 18.08.2019 09:30

Mathematics, 18.08.2019 09:30

Biology, 18.08.2019 09:30

History, 18.08.2019 09:30


Mathematics, 18.08.2019 09:30

English, 18.08.2019 09:30


Health, 18.08.2019 09:30

Mathematics, 18.08.2019 09:30

English, 18.08.2019 09:30

 .
.