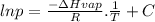Chemistry, 17.12.2019 21:31 lopeznadia838
The vapor pressure of a substance is measured over a range of temperatures. a plot of the natural log of the vapor pressure versus the inverse of the temperature (in kelvin) produces a straight line with a slope of −3.58×103k. what is the enthalpy of vaporization of the substance? 29.8 kj/mol2.32×1023kj/mol0.431 kj/mol294 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3


Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3
You know the right answer?
The vapor pressure of a substance is measured over a range of temperatures. a plot of the natural lo...
Questions

Mathematics, 04.03.2021 09:00


Mathematics, 04.03.2021 09:00

Mathematics, 04.03.2021 09:00

Mathematics, 04.03.2021 09:00


Mathematics, 04.03.2021 09:00

Mathematics, 04.03.2021 09:00


Mathematics, 04.03.2021 09:00


Mathematics, 04.03.2021 09:00


Mathematics, 04.03.2021 09:00

Biology, 04.03.2021 09:00

Mathematics, 04.03.2021 09:00



Mathematics, 04.03.2021 09:00