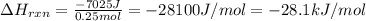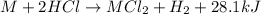Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted wit...

Chemistry, 17.12.2019 21:31 colestout2993
Consider the reaction: m + 2hcl → mcl2 + h2
when 0.25 mol of the metal, m, reacted with an aqueous hcl solution (the hcl is in excess), the temperature of the solution rose because the reaction produced 7025 j of heat. what is ∆h in kj per mol of m for this reaction? (hint: is this reaction exothermic or endothermic? )

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Questions





Engineering, 13.09.2021 18:40

Computers and Technology, 13.09.2021 18:40



Chemistry, 13.09.2021 18:40


Advanced Placement (AP), 13.09.2021 18:40





English, 13.09.2021 18:40

World Languages, 13.09.2021 18:40



History, 13.09.2021 18:40

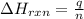
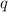 = amount of heat released = -7025 J
= amount of heat released = -7025 J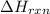 = enthalpy change of the reaction
= enthalpy change of the reaction