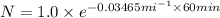Chemistry, 24.08.2019 01:30 svarner2001
The half-life of a radioactive isotope is 20.0 minutes. what is the total amount of a 1.00-gram sample of this sample of this isotope remaining after 1.00 hour?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
The half-life of a radioactive isotope is 20.0 minutes. what is the total amount of a 1.00-gram samp...
Questions

Mathematics, 21.10.2019 17:50




Mathematics, 21.10.2019 17:50


History, 21.10.2019 17:50

Mathematics, 21.10.2019 17:50



Mathematics, 21.10.2019 17:50


Mathematics, 21.10.2019 17:50

Social Studies, 21.10.2019 17:50



History, 21.10.2019 17:50


Mathematics, 21.10.2019 17:50

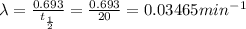

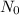 = initial amount = 1.0 g
= initial amount = 1.0 g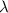 = rate constant
= rate constant