
Chemistry, 17.12.2019 22:31 missy922527
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) + cl2(g) pcl5(g) when 0.17 moles of cl2(g) are added to the equilibrium system at constant temperature: the value of kc the value of qc kc. the reaction must run in the forward direction to restablish equilibrium. run in the reverse direction to restablish equilibrium. remain the same. it is already at equilibrium. the concentration of pcl3 will

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Consider the following system at equilibrium where h° = -87.9 kj, and kc = 83.3, at 500 k. pcl3(g) +...
Questions


History, 08.11.2020 02:10

Spanish, 08.11.2020 02:10





Mathematics, 08.11.2020 02:20


Mathematics, 08.11.2020 02:20



Mathematics, 08.11.2020 02:20

Physics, 08.11.2020 02:20

History, 08.11.2020 02:20


Mathematics, 08.11.2020 02:20

Mathematics, 08.11.2020 02:20

Mathematics, 08.11.2020 02:20

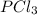 will decrease.
will decrease.

