
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the change in energy according to the law of conservation of energy.
a) there is a net gain of 185 kj of energy when decomposing hcl.
b) it takes more energy to break the bonds of hcl than form h2(g) + cl2(g).
c) the energy required to decompose hcl and produce h2(g) + cl2(g) are equal.
d) it takes less energy to break the bonds of hcl than form the bonds of h2(g) + cl2(g.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 14:30
The atomic number of an element is based on the number of electrons around its core mass of its nucleus number of protons in its nucleus number of neutrons in its nucleus
Answers: 2
You know the right answer?
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
Questions

Mathematics, 11.02.2020 19:00


Mathematics, 11.02.2020 19:00

History, 11.02.2020 19:00

Mathematics, 11.02.2020 19:00


Computers and Technology, 11.02.2020 19:01



Mathematics, 11.02.2020 19:01




History, 11.02.2020 19:02


English, 11.02.2020 19:03

Chemistry, 11.02.2020 19:04

History, 11.02.2020 19:04

Health, 11.02.2020 19:05

Mathematics, 11.02.2020 19:06

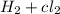 are equal.
are equal.

