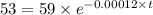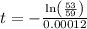The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 53.bq. the activity in a similar-sized sample of fresh wood is measured to be 59.bq.
1. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radio...
Questions

Biology, 22.10.2019 08:00

Mathematics, 22.10.2019 08:00

History, 22.10.2019 08:00

Mathematics, 22.10.2019 08:00

World Languages, 22.10.2019 08:00



World Languages, 22.10.2019 08:00



Mathematics, 22.10.2019 08:00





History, 22.10.2019 08:00

History, 22.10.2019 08:00

Mathematics, 22.10.2019 08:00


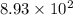 years
years
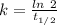
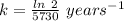
![[A_t]](/tpl/images/0423/2686/5262c.png) = 53 Bq
= 53 Bq![[A_t]=[A_0]e^{-kt}](/tpl/images/0423/2686/1ef89.png)