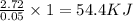Chemistry, 18.12.2019 01:31 mallorynichole19
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °c to 27.5 °c. calculate the enthalpy change for the reaction in kj per mol of hcl, assuming that the calorimeter loses only a negligible quantity of heat. the total volume of the solution is 100 ml, its density is 1.0 g/ml, and its specific heat is 4.18 j/g*k.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the tem...
Questions


Mathematics, 20.01.2021 09:50


Mathematics, 20.01.2021 09:50



Biology, 20.01.2021 14:00


English, 20.01.2021 14:00

English, 20.01.2021 14:00


English, 20.01.2021 14:00

Chemistry, 20.01.2021 14:00

English, 20.01.2021 14:00

English, 20.01.2021 14:00

Health, 20.01.2021 14:00


Mathematics, 20.01.2021 14:00

Mathematics, 20.01.2021 14:00

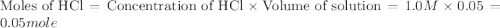
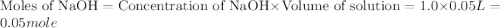

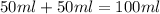
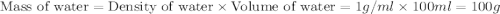
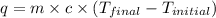
 = specific heat of water =
= specific heat of water = 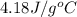
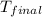 = final temperature of water =
= final temperature of water = 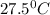
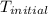 = initial temperature of metal =
= initial temperature of metal = 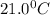
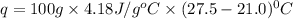
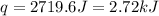
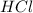 :
: