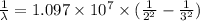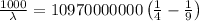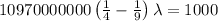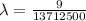Chemistry, 18.12.2019 01:31 tylermdons
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation, ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
a) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3 . round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -r...
e= -r...
Questions

Social Studies, 06.01.2021 16:20


Mathematics, 06.01.2021 16:20

Chemistry, 06.01.2021 16:20

Mathematics, 06.01.2021 16:20

French, 06.01.2021 16:20

Mathematics, 06.01.2021 16:20

Mathematics, 06.01.2021 16:20

Physics, 06.01.2021 16:20

Mathematics, 06.01.2021 16:20



Mathematics, 06.01.2021 16:20

German, 06.01.2021 16:20

Mathematics, 06.01.2021 16:20


Mathematics, 06.01.2021 16:20

Biology, 06.01.2021 16:20


Mathematics, 06.01.2021 16:20

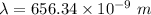
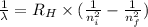
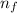 = 3
= 3
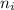 = 2
= 2