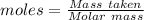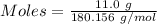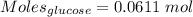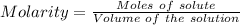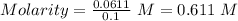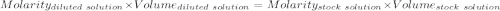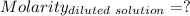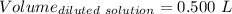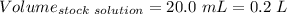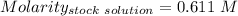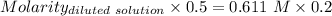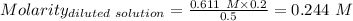Chemistry, 18.12.2019 01:31 joannsrods
(lo 4e, 4f) a student placed 11.0 g of glucose (c6h12o6) in a volumetric flask, added enough water to dissolve the glucose by swirling, then carefully added additional water until the 100. ml mark on the neck of the flask was reached. the flask was then shaken until the solution was uniform. a 20.0 ml sample of this glucose solution was diluted to 0.500l. what is the concentration of the dilute solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
(lo 4e, 4f) a student placed 11.0 g of glucose (c6h12o6) in a volumetric flask, added enough water t...
Questions

Mathematics, 03.11.2020 06:20

English, 03.11.2020 06:20


Mathematics, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20


Social Studies, 03.11.2020 06:20

History, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20

Chemistry, 03.11.2020 06:20



Mathematics, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20

Social Studies, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20

Social Studies, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20

Mathematics, 03.11.2020 06:20

English, 03.11.2020 06:20