Chemistry, 18.12.2019 02:31 gwendallinesikes
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is expanding from 8.00 to 24.0 l in volume at 1.00 atm. (remember that 101.3 j = 1 l·atm)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Calculate the change in internal energy (δe) for a system that is absorbing 35.8 kj of heat and is e...
Questions



Computers and Technology, 26.11.2019 21:31

English, 26.11.2019 21:31
















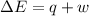
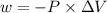
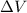 is the change in volume
is the change in volume
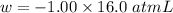
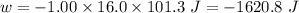 (work is done by the system)
(work is done by the system)