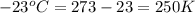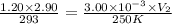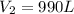Chemistry, 18.12.2019 03:31 ricardodeleon152
Agas-filled balloon with a volume of 2.90 l at 1.20 atm and 20°c is allowed to rise to the stratosphere (about 30 km above the surface of the earth), where the temperature and pressure are −23°c and 3.00 × 10−3 atm, respectively. calculate the final volume of the balloon.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Agas-filled balloon with a volume of 2.90 l at 1.20 atm and 20°c is allowed to rise to the stratosph...
Questions






History, 28.11.2019 06:31














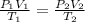
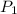 = initial pressure of gas = 1.20 atm
= initial pressure of gas = 1.20 atm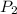 = final pressure of gas =
= final pressure of gas = 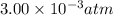
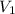 = initial volume of gas = 2.90 L
= initial volume of gas = 2.90 L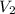 = final volume of gas = ?
= final volume of gas = ?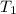 = initial temperature of gas =
= initial temperature of gas = 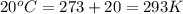
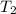 = final temperature of gas =
= final temperature of gas =